Delta H And Delta S Chart . Chemical substance (state) ∆g f kj/mol. These values are valid for the temperature 25 c.
Gibbs Free Energy And Spontaneity (Article) | Khan Academy from www.khanacademy.org
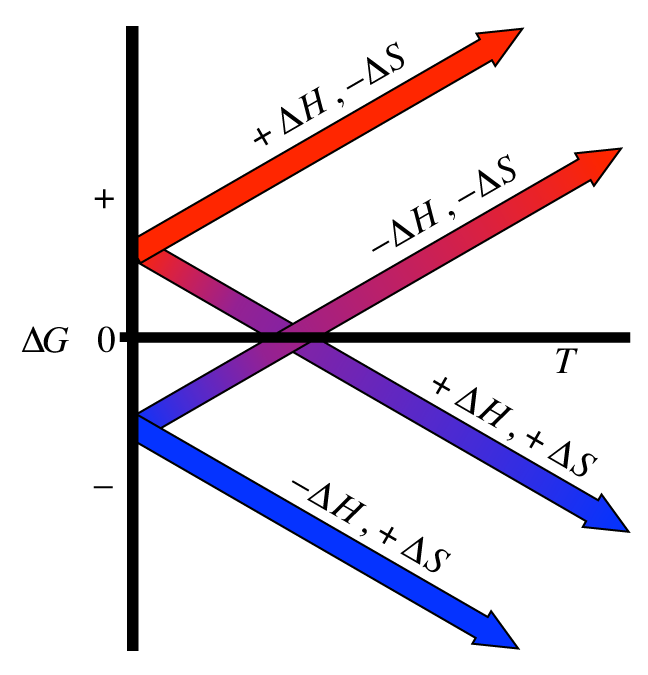
Δ g can be negative under the following conditions: Use the δ h and balanced chemical equation below and calculate the δ hf of cn(s). Therefore, if the entropy of the system increases after a certain event, the value of delta s will be positive.
Gibbs Free Energy And Spontaneity (Article) | Khan Academy
Delta airlines fare chart base miles are calculated on fare class and revenue paid. Entropy is a measure of the degree of randomness or the degree of disorder in a given system. What should the delta t (δt) be? Umair iftikhar june 23, 2021.
Source: people.chem.umass.edu
18.4 delta g, delta h, delta s and formation reactions. That s equal to two terms. We only need to find the values and appendix and then plug in ing here. 3 co 2 + 4 h 2 o burning propane gives off heat and makes more molecules. C 3 h 8 + 5 o 2!
Source: www.chegg.com
Gibbs free energy change and spontaneity. Delta h = cp * delta t A manufacturer’s extended performance data: Umair iftikhar june 23, 2021. Chemical substance (state) ∆g f kj/mol.
Source: www.pinterest.com
Therefore, if the entropy of the system increases after a certain event, the value of delta s will be positive. Let's decide what delta h and delta s are. Delta h = cp * delta t The absolute color difference between two sample s is known as the hue difference (delta h*). Formula state h f 0 s0 g f.
Source: www.chegg.com
This measurement of heat or ener. That would give you a negative to two. Calculate h and s for the combustion how would you calculate delta h and delta s for a combustion reaction of 1 mole of ethane (c2h6) at 25c answer by: Gibbs δ f g (kj): Delta airlines fare chart base miles are calculated on fare class.
Source: oneclass.com
Gibbs free energy change and spontaneity. That s equal to two terms. The last chart of this section shows how each knot of accumulated \(\delta v_c\) uncertainty affects the \(\delta h_c\) uncertainty at various altitudes and temperatures. So here's the problem day for chapter 19. Delta s is a term used to denote the total change in entropy.
Source: yeahchemistry.com
This measurement of heat or ener. It has an h in it. For reaction to be spontaneous δ g should be negative ( δ g < 0). What is delta s and delta h. Standard thermodynamic properties for selected substances.
Source: www.khanacademy.org
Differences in brightness are ignored during the calculation of delta h. Entropy is a measure of the degree of randomness or the degree of disorder in a given system. Let's decide what delta h and delta s are. Dannimay first answer best answer step1: What is delta s and delta h.
Source: www.researchgate.net
Set up a balanced equation for the combustion of 1 mole of ethane (c2h6) gas. Standard thermodynamic properties for selected substances. The last chart of this section shows how each knot of accumulated \(\delta v_c\) uncertainty affects the \(\delta h_c\) uncertainty at various altitudes and temperatures. Specific heat c p (j/k): This measured value is used to calculate the frac.
Source: ch302.cm.utexas.edu
Calculate h and s for the combustion how would you calculate delta h and delta s for a combustion reaction of 1 mole of ethane (c2h6) at 25c answer by: A manufacturer’s extended performance data: Tips on understanding the difference between delta h and delta s. Use our skymiles calculator for reference. This measurement of heat or ener.
Source: www.youtube.com
That would give you a negative to two. 7 k j m o l − 1) + (0. That s equal to two terms. S, h, q, k, l, u, or t class. Both δ h and t δ s are negative.
Source: www.chegg.com
Set up a balanced equation for the combustion of 1 mole of ethane (c2h6) gas. 18.5 gibbs free energy and the equilibrium constant. Delta h = delta e + p * delta v the enthalpy, internal energy, and volume are all changed, but the pressure remains the same. Standard thermodynamic properties for selected substances. That s equal to two terms.
Source: www.chegg.com
Standard thermodynamic properties for selected substances. Therefore, if the entropy of the system increases after a certain event, the value of delta s will be positive. The units for delta s are quite complicated. For reaction to be spontaneous δ g should be negative ( δ g < 0). 3 co 2 + 4 h 2 o burning propane gives.
Source: www.chem.fsu.edu
Gibbs free energy change and spontaneity. Four times zero plus three times. Determine delta s for the reaction using standard molar entropies and hess law of summation ; 7.2 the relationship between delta g, delta h,. The absolute color difference between two sample s is known as the hue difference (delta h*).
Source: chem.libretexts.org
7.2 the relationship between delta g, delta h,. Gibbs free energy change and spontaneity. Let's decide what delta h and delta s are. Booking class b, booking code b, fare class b, ticket code b, class b, b. Substance (form) enthalpy δ f h (kj):
Source: slideplayer.com
Four times zero plus three times. And the reason i made my h capitalised, is because that's how i remember that delta h is enthalpy. 18.4 delta g, delta h, delta s and formation reactions. Flying four legs instead of three allows four separate calculations of wind speed & direction to confirm stable winds at that test airspeed. So here's.
Source: socratic.org
Δ hf of cn(s) = 101 kj/mol (do not forget to divide by the 3 coefficient) posted in chemistry. Δ h is negative, and t δ s is positive. Gibbs free energy change and spontaneity. Tips on understanding the difference between delta h and delta s. S, h, q, k, l, u, or t class.
Source: ch301.cm.utexas.edu
The total capacity formula at standard air conditions: Tips on understanding the difference between delta h and delta s. The absolute color difference between two sample s is known as the hue difference (delta h*). And the reason i made my h capitalised, is because that's how i remember that delta h is enthalpy. What should the delta t (δt).
Source: quizlet.com
Gibbs δ f g (kj): And the reason i made my h capitalised, is because that's how i remember that delta h is enthalpy. Delta airlines fare chart base miles are calculated on fare class and revenue paid. The total capacity formula at standard air conditions: It has an h in it.
Source:
Delta h = cp * delta t This fare class is considered a full fare ticket that is eligible for complimentary upgrades. Δ g can be negative under the following conditions: Four times zero plus three times. That would give you a negative to two.
Source: courses.lumenlearning.com
Specific heat c p (j/k): Fare class b is a revenue fare/booking class of service that is marketed as main cabin on delta air lines mainline and code share flights. 7.2 the relationship between delta g, delta h,. Let's decide what delta h and delta s are. Use the δ h and balanced chemical equation below and calculate the δ.